Indications for antiviral therapy
Introduction
The decision to start antiviral therapy for hepatitis B is related to the phase of infection and the presence or absence of cirrhosis. This decision can be complex and should be made in consultation with a specialist or other practitioner accredited to treat hepatitis B.
Phases of chronic hepatitis B infection describes the natural history of chronic hepatitis B (including the four phases of infection), when treatment should be considered, and the frequency of monitoring in each phase. See below for further discussion about starting treatment in patients without cirrhosis and patients with cirrhosis.
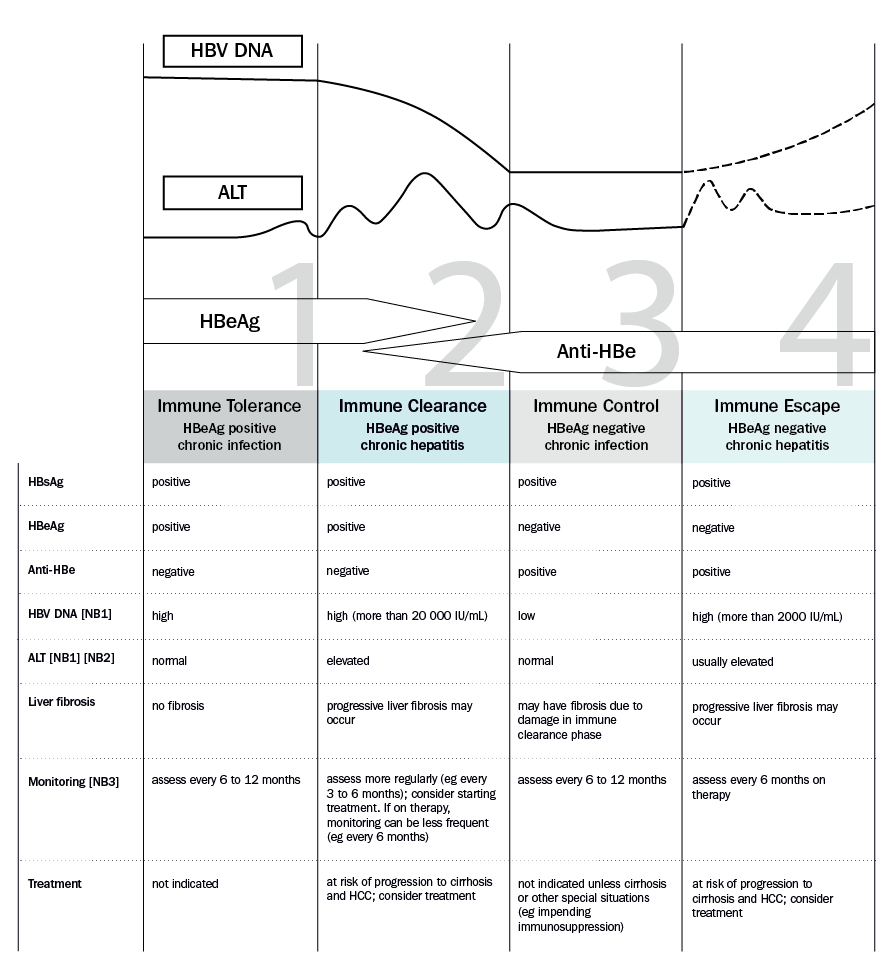
ALT = alanine aminotransferase; Anti-HBe = hepatitis B e antibody; HBeAg = hepatitis B e antigen; HBsAg = hepatitis B surface antigen; HBV = hepatitis B virus; HCC = hepatocellular carcinoma
NB1: The lines in the graph showing HBV DNA and ALT concentrations indicate trends only and are not depicted to scale. Actual concentrations are highly variable and can fluctuate over time in an individual.
NB2: Elevated ALT for women: more than 19 units/L; men: more than 30 units/L.
NB3: Monitoring includes measurement of HBV DNA viral load; this is rebatable on the Medicare Benefits Schedule (MBS) once per year for patients in the community not receiving antiviral therapy (or four times per year for those on treatment). Surveillance for hepatocellular carcinoma should be performed every 6 months in patients at high risk—see Surveillance for hepatocellular carcinoma in patients with chronic hepatitis B.
Adapted with permission from the Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine; Decision-making in HBV (2015). [URL]
Patients without cirrhosis
For patients who are not cirrhotic, antiviral therapy is indicated in the immune clearance (HBeAg positive chronic hepatitis) phase or immune escape (HBeAg negative chronic hepatitis) phase, if the ALT is persistently elevated (eg for more than 3 months) or there is evidence of liver fibrosisAustralian hepatitis B consensus statement 2022. These phases are characterised by active liver inflammation with risk of liver fibrosis progression, cirrhosis and hepatocellular carcinoma.
The approach in each phase is as follows:
- phase 1—immune tolerance (HBeAg positive chronic infection): monitor for progression to the immune clearance phase, including measurement of ALT and HBV DNA concentrations. Antiviral therapy is not generally indicated in this phase1
- phase 2—immune clearance (HBeAg positive chronic hepatitis): antiviral therapy is indicated for patients who have an HBV DNA viral load above 20 000 IU/mL and either a persistently elevated ALT2 (eg for more than 3 months) or evidence of liver fibrosis. These patients are usually treated with entecavir or tenofovir, or occasionally peginterferon—see Treatment regimens and Monitoring antiviral therapy. The goal of treatment is hepatitis B e antigen (HBeAg) seroconversion, which is usually associated with remission of disease. For information on duration of therapy, see Duration of treatment and stopping therapy
- phase 3—immune control (HBeAg negative chronic infection): monitor for progression to the immune escape phase, including measurement of ALT and HBV DNA concentrations. Antiviral therapy is not generally indicated in this phase
- phase 4—immune escape (HBeAg negative chronic hepatitis): antiviral therapy is indicated for patients who have an HBV DNA viral load above 2000 IU/mL and either a persistently elevated ALT2 (eg for more than 3 months) or evidence of liver fibrosis. The treatment of choice is entecavir or tenofovir—see Treatment regimens and Monitoring antiviral therapy. Treatment is usually long term; for information on duration of therapy, see Duration of treatment and stopping therapy.
Antiviral therapy may also be indicated in pregnancy and patients undergoing cancer chemotherapy or immunosuppression, regardless of the phase of disease.
Patients with cirrhosis
All patients with cirrhosis and detectable HBV DNA, regardless of their ALT concentration, should be started on entecavir or tenofovir. In patients with cirrhosis, treatment is typically lifelong—see Duration of treatment and stopping therapy for more informationAustralian hepatitis B consensus statement 2022.
