Choosing an antiepileptic drug
The aim of drug therapy for epilepsy is to completely suppress seizures without adverse effects. This is not always possible and a compromise may be needed. A general approach to the initial management of tonic-clonic seizures is summarised in Initial management of tonic-clonic seizures.
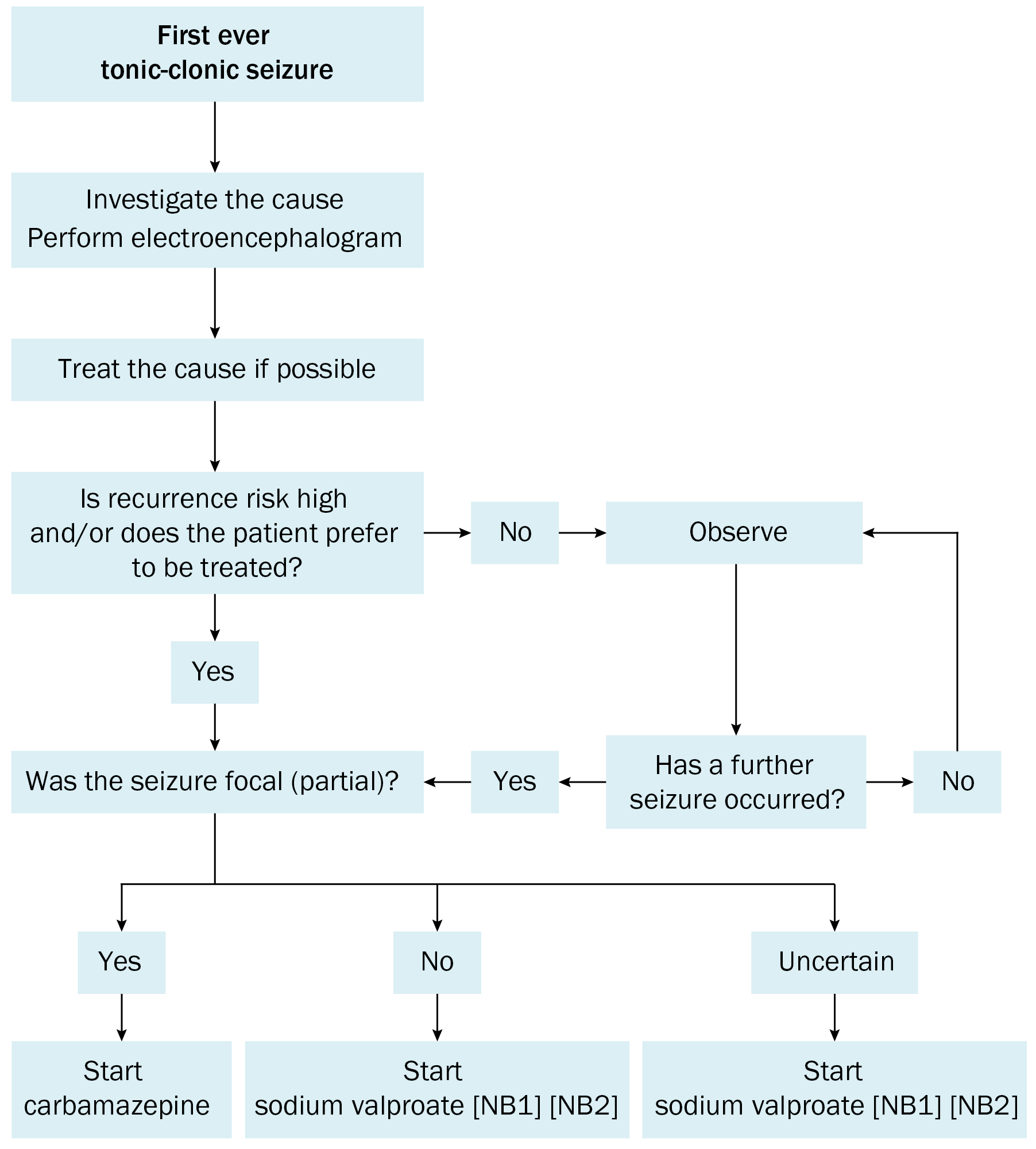
NB1: If possible, avoid sodium valproate in females of childbearing potential; see Planning for pregnancy in patients with epilepsy and Teratogenic and neurodevelopmental effects of antiepileptic drugs. If sodium valproate is the drug of choice, ensure reliable contraception.
NB2: If considering starting sodium valproate in males of reproductive potential, see Sodium valproate use in males of reproductive potential; a discussion about the potential risk of neurodevelopmental disorders in children born to males taking sodium valproate may be appropriate.
Many factors are considered when choosing the most appropriate drug for the patient. Based on efficacy, the first-line drug for focal (partial) epilepsy is carbamazepine, and for generalised epilepsy is sodium valproate. Factors that influence the choice of drug are listed in Factors affecting choice of antiepileptic drug.
It is essential to discuss plans for pregnancy with female patients of childbearing age. If possible, avoid sodium valproate in these patients. If sodium valproate is the drug of choice, ensure reliable contraception. Before starting valproate therapy, discuss the harms and benefits, see Planning for pregnancy in patients with epilepsy and Teratogenic and neurodevelopmental effects of antiepileptic drugs.
If considering starting sodium valproate in males of reproductive potential, see Sodium valproate use in males of reproductive potential; a discussion about the potential risk of neurodevelopmental disorders in children born to males taking sodium valproate may be appropriate.
Factors to consider when choosing an antiepileptic drug include:
- efficacy in treating the syndrome
- certainty of syndrome diagnosis—if uncertain, consider drugs that are effective in focal (partial) and generalised epilepsies (eg sodium valproate, levetiracetam, lamotrigine, topiramate)
- females of childbearing potential and pregnancy—if possible, avoid starting sodium valproate in females of childbearing potential (see Planning for pregnancy in patients with epilepsy and Teratogenic and neurodevelopmental effects of antiepileptic drugs)
- males of reproductive potential—if considering sodium valproate, see Sodium valproate use in males of reproductive potential; a discussion about the potential risk of neurodevelopmental disorders in children born to males taking sodium valproate may be appropriate
- adverse effects
- body weight changes (eg sodium valproate and pregabalin can cause weight gain; topiramate can cause weight loss)
- impaired cognition (eg phenobarbital [phenobarbitone] and topiramate are more likely to cause sedation or cognitive impairment, which may be overlooked if the patient already has cognitive impairment; benzodiazepines and phenytoin are more likely to cause sedation in children)
- cosmetic changes (eg phenytoin can cause hirsutism, gingival hyperplasia and coarser facial features; sodium valproate can cause hair loss)
- hypersensitivity (eg patients of Asian origin [other than Japanese] are at higher risk of serious skin reactions caused by carbamazepine and phenytoin)
- age (eg sodium valproate hepatotoxicity is more likely in infants)
- cost
- ease of use
- need for measuring serum drug concentration
- pharmacokinetics
- drug interactions (eg with contraceptives or warfarin)
- time to achieve therapeutic dose (eg phenytoin can be started at therapeutic doses; lamotrigine, perampanel and topiramate need to be started slowly)
- preparations available (eg intravenous, paediatric, liquid, scored)
There is anecdotal evidence that cannabis-derived products may be beneficial in several epilepsy syndromes. A single randomised controlled trial1 showed modest efficacy in Dravet syndrome. It is premature to recommend the use of medicinal cannabis in other epilepsy syndromes before the results of properly conducted clinical trials are known.
There is little evidence that switching between originator and generic brands or between generic brands of antiepileptic drugs is harmful.
