Overview
Drug treatment for asthma in adults and adolescents is introduced in a stepwise manner, as shown in Stepped approach to starting and adjusting asthma therapy in adults and adolescents. For details about each step, including drug dosages, see the relevant sections—Step 1, Step 2, Step 3, Step 4 and Step 5.
Patients presenting with severe uncontrolled asthma may need a short course of oral corticosteroids, or to start therapy at a higher step (eg with medium- or high-dose inhaled corticosteroid [ICS] plus long-acting beta2 agonist [LABA]), but this is not common. See also Acute asthma for advice about treating acute presentations.
Patients who remain uncontrolled despite optimised therapy (including management of comorbidities and other contributing factors), are considered to have severe asthma. Specialist assessment (eg phenotypic assessment) and treatment may be required—see Severe asthma in adults and adolescents: specialist management.
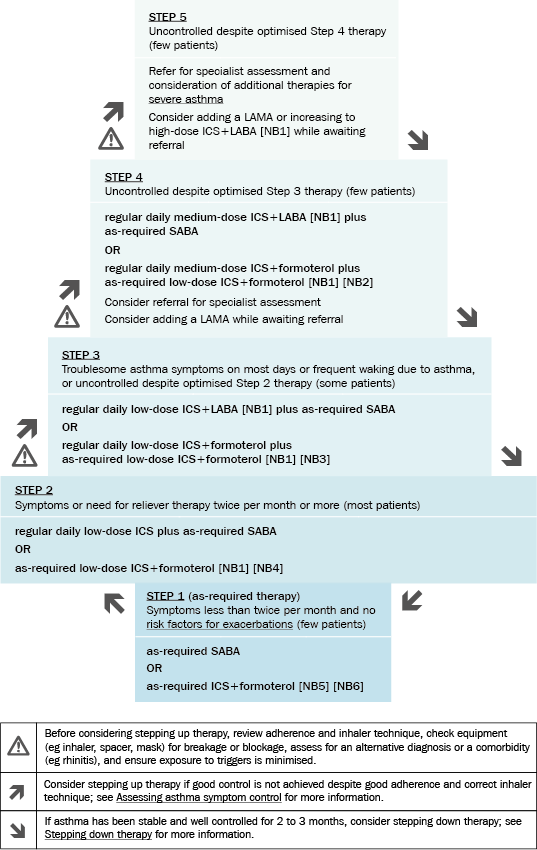
ICS = inhaled corticosteroid; LABA = long-acting beta2 agonist; LAMA = long-acting muscarinic antagonist; SABA = short-acting beta2 agonist
NB1: Always give ICS+LABA therapy as a combination inhaler to avoid the possibility of patients taking a LABA without an ICS; LABA monotherapy increases the risk of exacerbations and asthma-related death.
NB2: At the time of writing, the only combination product approved for use as Step 4 medium-dose maintenance and reliever therapy is budesonide+formoterol. Beclometasone+formoterol can only be used as maintenance and reliever therapy for Step 3 low-dose therapy; it is not approved for Step 4 medium-dose maintenance and reliever therapy.
NB3: At the time of writing, the only combination products approved for use as Step 3 low-dose maintenance and reliever therapy are beclometasone+formoterol and budesonide+formoterol. Beclometasone+formoterol can only be used as maintenance and reliever therapy for Step 3 low-dose therapy; it is not approved for Step 4 medium-dose maintenance and reliever therapy.
NB4: At the time of writing, the only combination product approved for use as Step 2 as-required therapy is budesonide+formoterol.
NB5: No clinical trials have evaluated the effects of as-required budesonide+formoterol versus as-required SABA in patients with asthma symptoms less than twice per month and no waking due to asthma, and without risk factors for exacerbations (eg flare ups that required oral corticosteroids in the previous 12 months).
NB6: At the time of writing, the only combination product approved for use as Step 1 as-required therapy is budesonide+formoterol.
Adapted from the Australian Asthma Handbook © 2020 National Asthma Council Australia. Accessed 31 August 2020.
