Overview of stepwise therapy for children 1 to 5 years
A definitive diagnosis of asthma is not possible in children aged 1 to 5 years, so drug therapy should always be considered a trial, with continuous reassessment to determine the need for ongoing treatment. For detailed information about assessment of wheeze and asthma in children aged 1 to 5 years, including guidance on the clinical assessment and decision to trial treatment, see Acute wheeze and assessment of asthma in children 1 to 5.
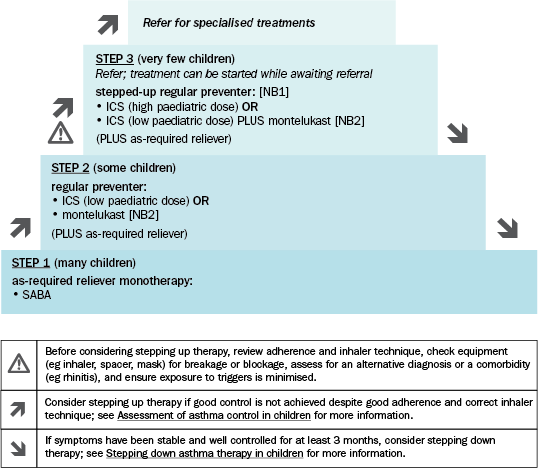
Treatment of wheeze in children 1 to 5 years is often not necessary, particularly if symptoms are not bothersome (eg do not limit activity or affect sleep). For recurrent bothersome symptoms associated with increased work of breathing, short-term Step 1 therapy with an as-required short-acting beta2 agonist (SABA) (salbutamol) is usually sufficient. A small number of children with frequent or severe symptoms (eg requiring emergency-department visits) that occur with viral respiratory tract infections, or with symptoms between infections, may benefit from Step 2 therapy with a regular preventer. Step 3 therapy is required for very few children. Therapy is summarised in Stepped approach to starting and adjusting therapy for wheeze and asthma in children 1 to 5 years old. See each step for detailed information about indications, treatment options and drug doses.
ICS = inhaled corticosteroid; LABA = long-acting beta2 agonist; SABA = short-acting beta2 agonist
NB1: Use of LABAs in children younger than 5 years is not supported by evidence; they are not recommended in this age group.
NB2: Montelukast is less effective than ICS and has been associated with neuropsychiatric adverse effects (eg behavioural changes, depression, suicidality). Adverse effects are generally mild and may be coincidental; however, symptoms may be serious and continue if treatment is not stopped. Advise parents and carers to be alert for changes in behaviour and new psychiatric symptoms. Stop treatment if these effects occur. In some cases, symptoms may persist after stopping treatment; patients should be monitored and provided supportive care until symptoms resolve. See the Australian Therapeutic Goods Administration (TGA) safety alert for more information.
Adapted from the Australian Asthma Handbook © 2020 National Asthma Council Australia. Accessed 31 August 2020.
Review symptom control 4 to 6 weeks after starting therapy to determine whether good control has been achieved. A continuous cycle of reviewing response and adjusting therapy (stepping up or down) is recommended, aiming to achieve a normal quality of life using the minimal dose regimen. See Review and ongoing management of asthma in children.
