Clinical history for initial assessment of patients reporting penicillin hypersensitivity
Clinical history is critically important in the diagnosis of antimicrobial hypersensitivity. Information about obtaining an accurate antimicrobial hypersensitivity history can be found in Clinical history for initial assessment of patients reporting antimicrobial hypersensitivity.
For penicillin allergy, clinical decision tools have been developed to further risk assessmentStevenson 2020Trubiano 2020. Two validated allergy assessment tools are PEN-FAST Penicillin Allergy Decision Rule and the Beta-lactam antibiotic allergy assessment tool. These are predominantly used by specialists with expertise in drug allergy (eg allergists, infectious diseases physicians).
PEN-FAST is a clinical decision rule that has been locally and internationally validated for penicillin allergy risk assessment; one in 100 patients who had a PEN-FAST score less than 3 had a confirm penicillin allergy when tested. This tool is validated for use in adults and may be useful in adolescents, but is not useful for penicillin allergy risk assessment in childrenCopaescu 2022.
The Beta-lactam antibiotic allergy assessment tool has been validated in adults and children and can be used by a range of clinicians with no specific allergy training (eg nurses, pharmacists). This tool can also be used to determine the severity of the penicillin hypersensitivity reactionDevchand 2019.
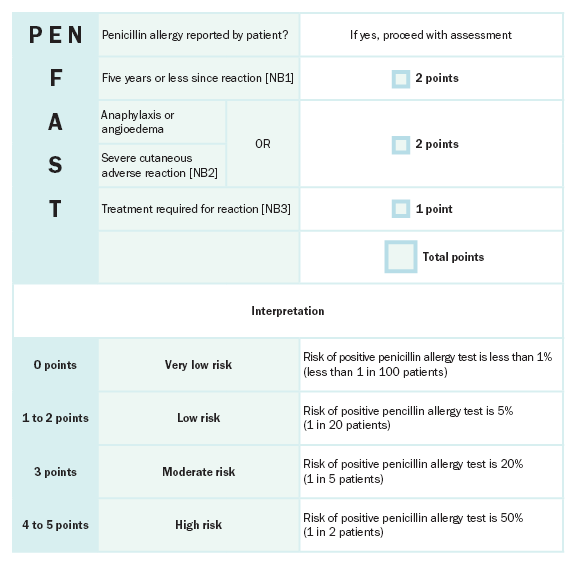
NB1: If the time since the reaction is unknown, give the patient zero points.
NB2: Severe delayed cutaneous reactions include drug rash with eosinophilia and systemic symptoms [DRESS], Stevens–Johnson syndrome / toxic epidermal necrolysis [SJS/TEN], and acute generalised exanthematous pustulosis (AGEP). Patients reporting a severe delayed rash with mucosal involvement should be assumed to have a severe cutaneous adverse reaction even if it has not been clinically confirmed. In the validation study, acute interstitial nephritis, drug-induced liver injury (DILI), serum sickness and isolated drug fever were excluded phenotypes from the derivation and validation cohorts.
NB3: If the requirement for treatment is unknown, give the patient one point.
Reproduced with permission from JAMA Internal Medicine, Development and Validation of a Penicillin Allergy Clinical Decision Rule. 2020. 180(5): 10.1016/j.jaip.2018.07.048. Copyright © (2020) American Medical Association. All rights reserved, including those for text and data mining, AI training, and similar technologies. [URL]
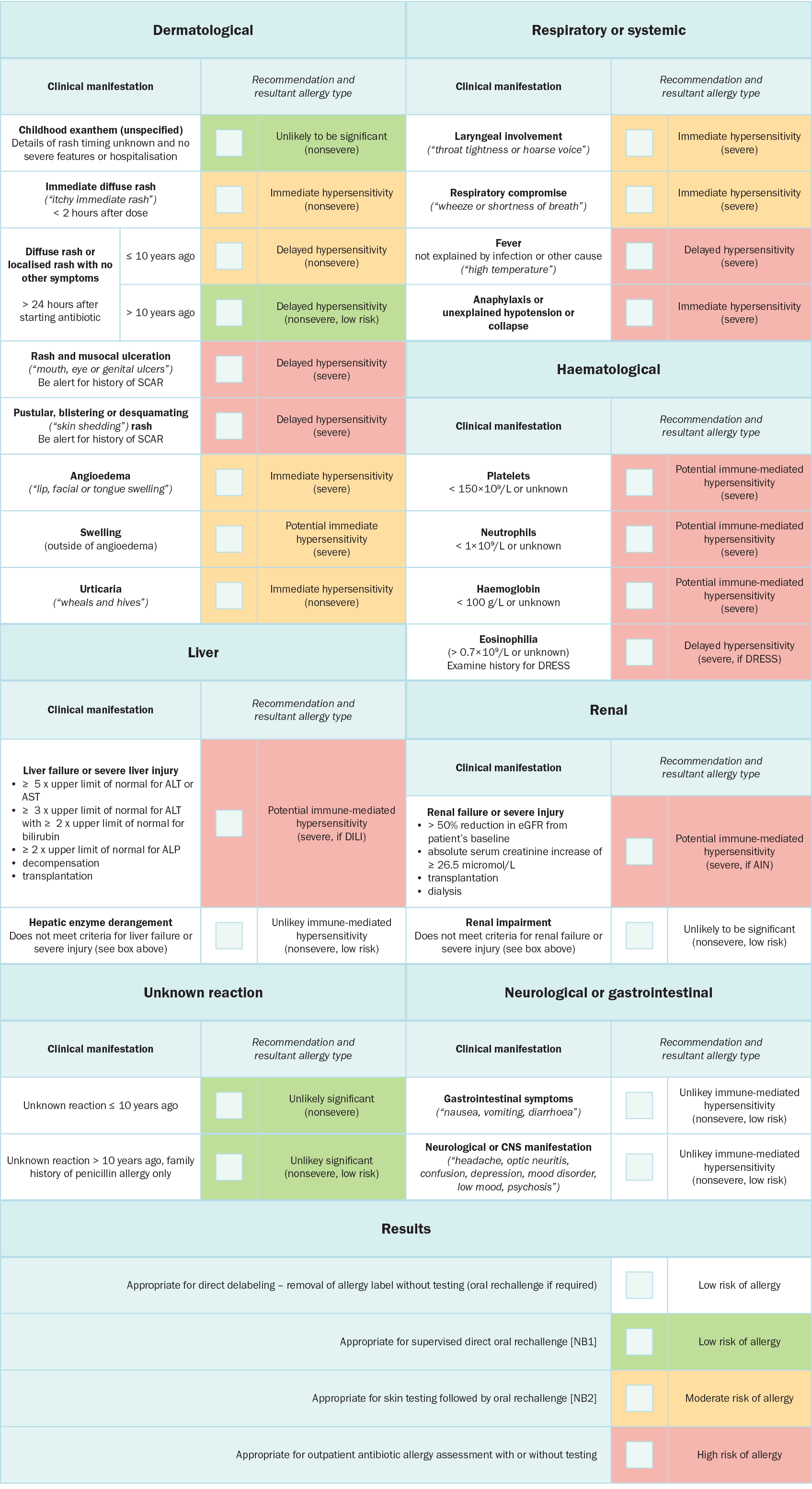
AIN = acute interstitial nephritis; ALP = alkaline phosphatase; ALT = alanine transaminase; AST = aspartate aminotransferase; CNS = central nervous system; DILI = drug-induced liver injury; DRESS = drug reaction with eosinophilia and systemic symptoms; eGFR = estimated glomerular filtration rate; SCAR = severe cutaneous adverse drug reactions; ULN = upper limit of normal
NB1: In the appropriate setting, a direct oral rechallenge (ie oral rechallenge without prior skin testing) may be performed under specialist guidance.
NB2: Skin testing followed by oral rechallenge can be performed in the outpatient or inpatient setting. Patients with features of severe life-threatening allergy should have allergy testing performed in the outpatient setting (rather than inpatient setting) to ensure a detailed collateral history is obtained, that there is specialist allergy oversight and that there is resolution of acute illness. If these requirements can be achieved in the inpatient setting, then testing may proceed in this setting with caution.
Reproduced from The Journal of Allergy and Clinical Immunology: In Practice, Vol 7/edition 3, Devchand M, Urbancic KF, Khumra S, Douglas AP, Smibert O, Cohen E, Sutherland, M, Phillips, EJ, Trubiano, JA, Pathways to improved antibiotic allergy and antimicrobial stewardship practice: The validation of a beta-lactam antibiotic allergy assessment tool, 1063-5, Copyright (2024), with permission from Elsevier [URL].
